Solution
A perfectly homogenous mixture of two or more components is called as solution. By homogenous mixture we mean that its composition and properties are uniform throughout the mixture. Solution is a mixture not compound, as its composition is variable within certain limits and a solution exhibits all the chemical properties of its components. A solution has two essential components i.e. solute and solvent. Generally, the component which is present in larger amounts is known as solvent. Solvent determines the physical state in which solution exists. One or more components present in the solution other than the solvent ( present in lesser amounts) or whose physical state is changed during the formation of solution is termed as solute.
Solution = Solute + Solvent
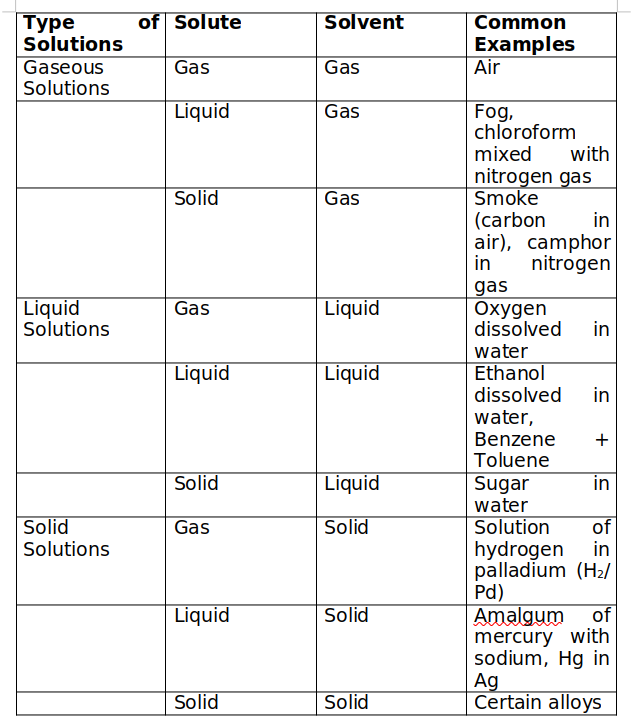
Binary Solutions
When only two components are present in the solution, it is called binary solution.
Ternary solutions are those in which three components are present and in Quarternary solutions, four components are present.
Different types of Binary Solutions
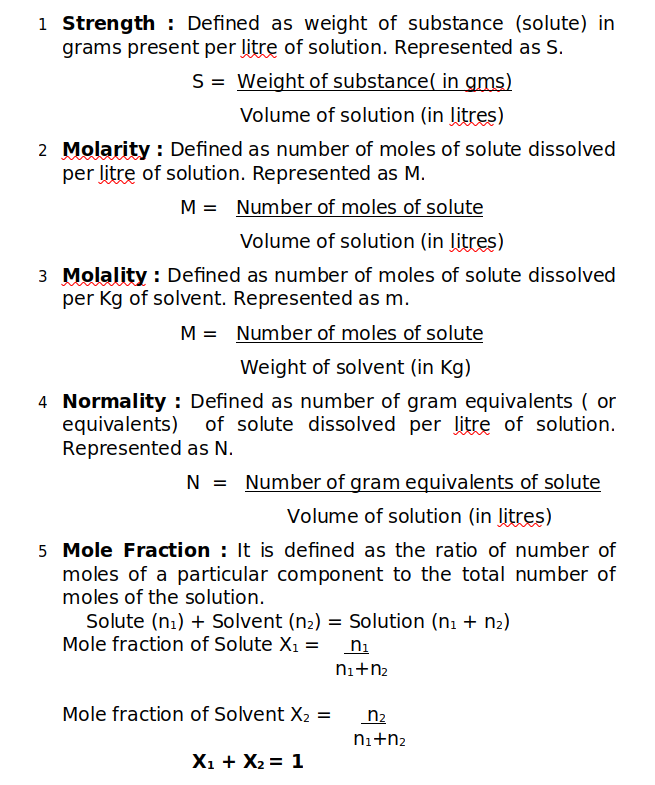
Concentration Terms
Composition of a solution can be described by expressing the concentration. There are many different ways (or terms) which are used to measure the concentration of solutions (quantitatively). These are

SOLUBILITY
Solubilty of a substance is defined as the maximum amount of solute that can be dissolved in a specified amount of solvent at a particular temperature.
Saturated solutions are those in which the amount of solute dissolved is equal to its solubility. That means if excess solute is dissolved in the solution, it would be in equilibrium with the solution.
Unsaturated solution is the one in which the amount of solute dissolved is less than its solubility at a particular temperature and more solute can be dissolved in it.
Super saturated solutions are those in which it is possible to dissolve more solute than its solubility, but under some special conditions. Super saturated solution is unstable system (i.e. metastable) and on adding crystal of solute or by agitating the solution, super saturated solution gets converted into the saturated solution.
Factors affecting Solubility :
The concentration of a saturated solution i.e. the solubility of a solute depends upon the following factors :
- Nature of solute and solvent
- Presence of other solutes
- Temperature
- Pressure
- Nature of solute and solvent :
A well known generalization regarding dissolution process is “ Like dissolves Like “. The same applies here. A polar solute has higher solubility in polar solvents while non-polar solute dissolves easily in non-polar solvents. For this reason polar solvent H2O is better solvent for polar solutes but poor solvent for non-polar solutes. In general, ionic solids are more soluble in polar solvents like water (or in other solvents having high dielectric constant )
- Presence of other solutes :
The solubility of a solute may be slightly or greatly affected by the presence of other solutes in the solution. If the solute already present has an ion in common, then solubility of solute added is greatly reduced. For example : solubility of NaCl is appreciably lowered in presence of KCl.
- Temperature :
Temperature is an important factor that can affect the solubility of almost all the substances.
a. Effect on the solubility of Gases : As a general rule, the solubility of a gas in water decreases as the temperature is raised. However there are some exceptions , there are some cases in which solubility first decreases and then increases as the temperature is increased . for example : solubility of He first decreases with the rise in temperature then increases. In some other liquid solvents, the solubility of gases may increase with increase in temperature.
b. Effect on the solubility of Solids and Liquids :
In most (not all) of the cases, the solubility of a solid substance increases with increase in temperature, but in some cases, it decreases with increase in temperature. It is better described by Le-Chatelier’s principle, according to which for endothermic dissolution, solubility must increase with increase in temperature while for exothermic dissolution, solubility decreases with increase in temperature. However, it is best to determine the effect of temperature on solubility experimentally.
- Pressure :
The solubilities of solids and liquids are not affected by external pressure. But for gases, the solubilities are greatly affected by pressure. It is better described under the heading Henry’s Law.
COLLIGATIVE PROPERTIES
Those properties of solution of non-volatile solutes, whose value depend upon the number of solute particles only and not upon their nature, are known as colligative properties. They also depend upon the association or dissociation of solute in solution. There are four important colligative properties , which are as follows :
- Osmotic pressure
- Lowering in vapour pressure
- Elevation in boiling point
- Depression in freezing point
- OSMOSIS AND OSMOTIC PRESSURE
Osmosis can be defined as “spontaneous net flow of solvent molecules from dilute solution (or from pure solvent) to concentrated solution when the two are separated through a semi-permeable membrane”. Osmosis is a bilateral process. The solvent molecules move in both the directions, however the net flow is from dilute solution to concentrated solution. Osmosis continues till the concentrations of two solutions become almost same.
Types of Osmosis : Osmosis occurs in many chemical and biological processes . There are two types of osmosis : exo-osmosis and endo-osmosis.
Exo-Osmosis : It involves the outward flow of solvent molecules. For example- when a grape is kept in concentrated sugar solution, it shrinks.
Endo-Osmosis : It involves the inward flow of solvent molecules. For example- when a dried resin is kept in water, it swells up.
OSMOTIC PRESSURE
In simple words, it is the pressure required to stop osmosis. Osmotic pressure of a solution is equal to the minimum external pressure applied on solution side just to prevent osmosis when solution is separated from pure solvent through a semi-permeable membrane. Osmotic pressure is a colligative property as it depends on the number of solute molecules and not on their identity.

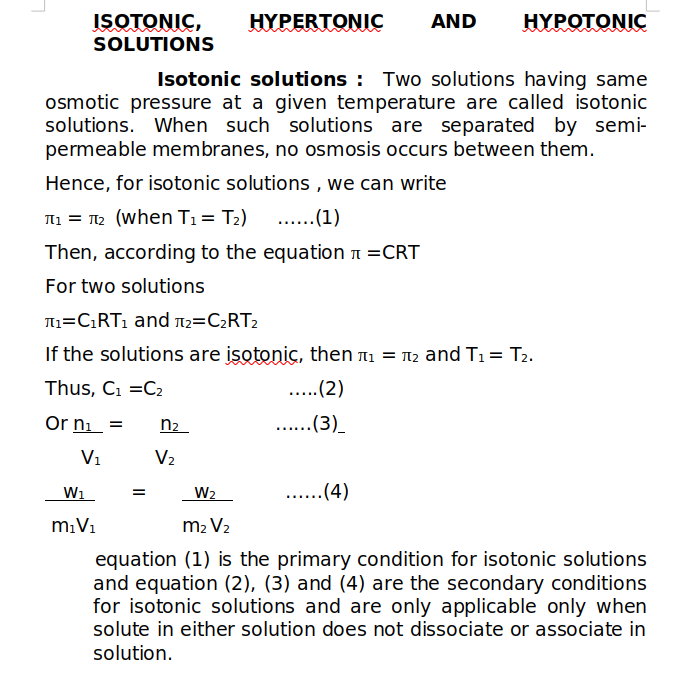
When osmotic pressure of two solutions are compared at same temperature , the solution whose osmotic pressure is less is called Hypotonic solution and the solution with high osmotic pressure is called as Hypertonic solution. A hypertonic solution has higher concentration and when the two solutions are separated by a semi-permeable membrane, the net flow of solvent particles is from hypotonic solution (less concentrated) to hypertonic solution (more concentrated).
REVERSE OSMOSIS :
The direction of osmosis can be reversed if a pressure larger than the osmotic pressure is applied to the solution side. That means, now the pure solvent flows out of the solution through the semi-permeable membrane. The phenomenon is called Reverse Osmosis and is of great practical utility. Reverse osmosis is used in the desalination of sea water. When pressure more than osmotic pressure is applied, pure water is squeezed out of the sea water through the membrane. The pressure required for the reverse osmosis to take place is quite high. The osmotic pressure of sea water is about 30 atm- this is the pressure that must be applied to the saline solution in order to stop the flow of water into the saline water from the fresh water (pure solvent). If the pressure on the salt solution is increased beyond 30 atm, the osmotic flow would be reversed, and fresh water would actually pass out from the salt solution to the other compartment of fresh water through the membrane. Desalination by reverse osmosis is considerably cheaper than distillation and avoids the technical difficulties associated with freezing. The main obstacle to this method is the development of a membrane that is permeable to water but not to other dissolved substances and that can be used on a large scale for prolonged durations under high pressure conditions. Once this problem is solved, then reverse osmosis could become a major desalination technique.
- LOWERING IN VAPOUR PRESSURE
Vapour Pressure
Consider a binary solution of two volatile liquids kept in a closed vessel. Molecules of both the liquids start to evaporate and gets converted to vapour molecules. These vapour molecules gets collected in the closed space above liquid and some of them regains liquid state (condense) when they strike on the liquid surface. In a closed container, process of evaporation and condensation occurs simultaneously. Initially, rate of condensation is zero, but it increases with time however the rate of evaporation remains constant. After sometime, the rates of these two opposing processes become same and system attains a liquid-vapour equilibrium. These vapours exert pressure on liquid surface and the pressure exerted by vapour molecules on the surface of liquid when they are in dynamic equilibrium with each other is known as Vapour Pressure.
When a non-volatile solute is added in a pure solvent its vapour pressure decreases, i.e., the vapour pressure of a solution is always less than its solvent (in pure state). This is known as lowering in vapour pressure. It occurs due to reduction in the relative surface area of liquid available for evaporation as a part of surface is occupied by non-volatile solute particles.

RAOULT՚S LAW
The French chemist, Francois Marte Raoult (1886) gave a relation to calculate the vapour pressure of solutions. According to this law, “ the vapour pressure of a solution is equal to the sum of partial vapour pressures of its constituents in the solution”. The partial vapour pressure of a component is equal to its vapour pressure in pure state multiplied by its mole fraction in solution.
Limitations of RAOULT՚S LAW
- It is not valid if solute reacts with solvent chemically.
- It is not valid if solute associates or dissociates in solution.
IDEAL AND NON-IDEAL SOLUTIONS :
Liquid-liquid solutions can be classified into ideal and non-ideal solutions on the basis of Raoult՚s law.
IDEAL SOLUTIONS
The solutions which obey Raoult՚s law over the entire range of concentration are known as ideal solutions. A solution is said to be ideal when it has following characteristics :
- ΔHmix = 0, i.e., heat is neither absorbed nor evolved during the formation of solution.
- ΔVmix = 0, i.e., the volume of solution would be equal to the sum of volumes of the two components.
- If the intermolecular attractive forces between solute-solute and solvent-solvent molecules are nearly equal to those between solute-solvent molecules, this leads to the formation of ideal solution.
Theoretically, a solution can not be perfectly ideal , however, liquid pairs having similar structure, polarity and chemical nature tend to behave almost ideally. Some solutions that are nearly ideal in behaviour are :
- CCl4 and SiCl4
- n-hexane and n-heptane
- Benzene and toluene
- Chlorobenzene and bromobenzene
- Methanol and ethanol
- Chloroethane and bromoethane etc.
NON-IDEAL SOLUTIONS
Solutions which do not obey Raoult՚s law over the entire range of concentration are known as non-ideal solutions. The vapour pressure of such a solution is either higher or lower than that predicted by Raoult՚s law. On the basis of nature of deviation, non-ideal solutions may be of two types :
- Solutions with positive deviation
- Solutions with negative deviation
- Solutions with positive deviation
Solution exhibits positive deviation if the experimental vapour pressure of solution is greater than as calculated by Raoult՚s law theoretically, at all compositions. i.e.,
V.P.exp >V.P.Th
For these solutions
(a). ΔHmix = +ve, i.e., formation of solution is endothermic process, that means heat is absorbed during the formation of solution.
(b). ΔVmix = +ve, i.e., the volume of solution would be greater than the sum of volumes of the two components.
( c). The cause for these deviations lies in the nature of interactions at the molecular level. In case of positive deviation from Raoult՚s law, solute-solvent interactions are weaker than those between solute-solute and solvent-solvent molecules. This is the reason why formation of such solutions is endothermic , and it also leads to the rise in vapour pressure and volume.
(d). Some examples of liquid pairs which show positive deviation are :
(i). Mixtures of ethanol and acetone.
In pure ethanol, molecules are hydrogen bonded. On adding acetone, its molecules get in between the ethanol molecules and break some of the hydrogen bonds between them. Due to weakening of interactions, the solution shows positive deviation from Raoult՚s law.
(ii). Solution of carbon disulphide and acetone
(iii). Benzene and acetone
(iv). Ethanol and water
(v). Ethanol and benzene
(vi). Ethanol and chloroform
(vii). Ethanol and cyclohexane etc.
- Solutions with negative deviation
Solution exhibits negative deviation if the experimental vapour pressure of solution is less than as calculated by Raoult՚s law theoretically, at all compositions. i.e.,
V.P.exp <V.P.Th
For these solutions
(a). ΔHmix = -ve, i.e., formation of solution is exothermic process, that means heat is evolved during the formation of solution.
(b). ΔVmix = -ve, i.e., the volume of solution would be less than the sum of volumes of the two components.
( c). In case of negative deviation from Raoult՚s law, solute-solvent interactions are stronger than those between solute-solute and solvent-solvent molecules. It explains why formation of such solutions is exothermic , and it also leads to the fall in vapour pressure and volume.
(d). Some examples of liquid pairs which show negative deviation are :
(i). Mixture of phenol and aniline
In this case, the intermolecular hydrogen bonding between phenolic proton and the lone pair on nitrogen atom of aniline is stronger than the respective intermolecular hydrogen bonding between similar molecules.
(ii). Mixture of chloroform and acetone
This is because chloroform molecule is able to form hydrogen bond with acetone molecule. This decreases the escaping tendency of molecules for each component and consequently the vapour pressure decreases.
(iii). Pyridine (C5H5N) and Acetic acid
(iv). Pyridine (C5H5N) and Formic acid
(v). Benzene and chloroform
(vi). Diethyl ether and chloroform
(v). Water and nitric acid
(vii). Aniline and acetone etc.
AZEOTROPES
Most pairs of liquids do not form ideal solutions. Some liquids on mixing, form azeotropes , which are binary mixtures having the same composition in liquid and vapour phase and boil at a constant temperature. In such cases, it is not possible to separate the components by fractional distillation.
Two types of azeotropes are :
Minimum boiling azeotrope and Maximum boiling azeotrope
In an ideal solution which obeys Raoult՚s law, there is no maximum or minimum in either the vapour pressure composition graphs or the temperature composition graphs. However, if there are strong negative deviations from Raoult՚s law, there is a minimum in the vapour pressure composition diagram and the maximum in the boiling point composition diagram. A solution with composition corresponding to the maximum boiling point is called a _maximum boiling azeotrope. _Nitric acid and water is an example of this class of azeotrope. This azeotrope has the approximate composition , 68% nitric acid and 32% water by mass, with a boiling point of 393.5K.
In case of positive deviations from Raoult՚s law, there is a maximum in the vapour pressure composition diagram and a minimum in the boiling point composition diagram. A solution with composition corresponding to the minimum boiling point is called a _minimum boiling azeotrope. _for example , ethanol-water mixture (obtained by fermentation of sugars) on fractional distillation gives a solution containing approximately 95% by volume of ethanol. Once this azeotrope composition has been achieved, the liquid and vapour have the same composition, and no further separation occurs.